On August 21, an expert panel of the Ministry of Health, Labor, and Welfare (MHLW) approved the manufacturing and marketing approval in Japan for Lecanemab (brand name Leqembi), a new treatment for Alzheimer’s disease co-developed by Japanese pharmaceutical giant Eisai and U.S. company Biogen. If officially approved, this will be the first therapeutic drug that eliminates the causative agent of dementia. The Minister of Health, Labor and Welfare will officially approve the drug in the future.
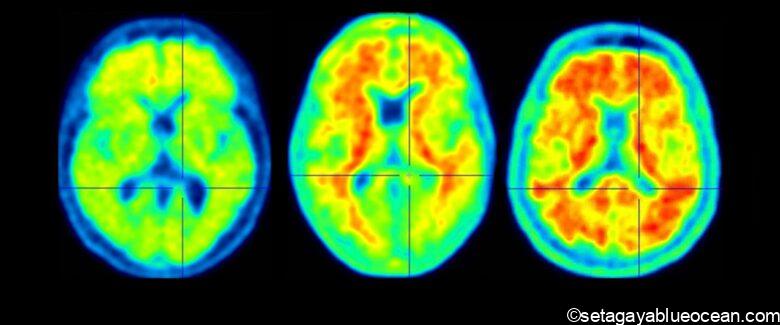
Alzheimer’s disease is a disease in which nerve cells in the brain break down and cognitive functions gradually decline.
It is believed to be caused by the accumulation of an abnormal protein called amyloid-β (Aβ) in the brain.
While existing dementia drugs work to alleviate symptoms by stimulating nerve function, Lecanemab aims to eliminate Aβ, the cause of the disease, and suppress the progression of the disease.
The drug targets people with mild dementia and mild cognitive impairment (MCI), a pre-dementia stage of the disease. Because it is difficult to regenerate damaged neurons, people with advanced symptoms are not eligible.
The drug is administered intravenously once every two weeks.
In an international clinical trial involving about 1,800 people, 18 months of administration of the drug reduced the deterioration of scores assessing the degree of memory and judgment by 27% compared to the sham drug. On the other hand, side effects were also identified, with edema in the brain reported in 12.6% of those who used the drug and microbleeding in 17.3%. In the U.S., it was recommended that before using Lecanemab, patients be tested to determine if they have a genotype that is known to be prone to side effects.
On the same day, the MHLW’s expert panel confirmed that genetic testing is not required in Japan, but that the government should check for bleeding in the brain after administration and warn people who are taking drugs that dissolve blood clots.

コメント